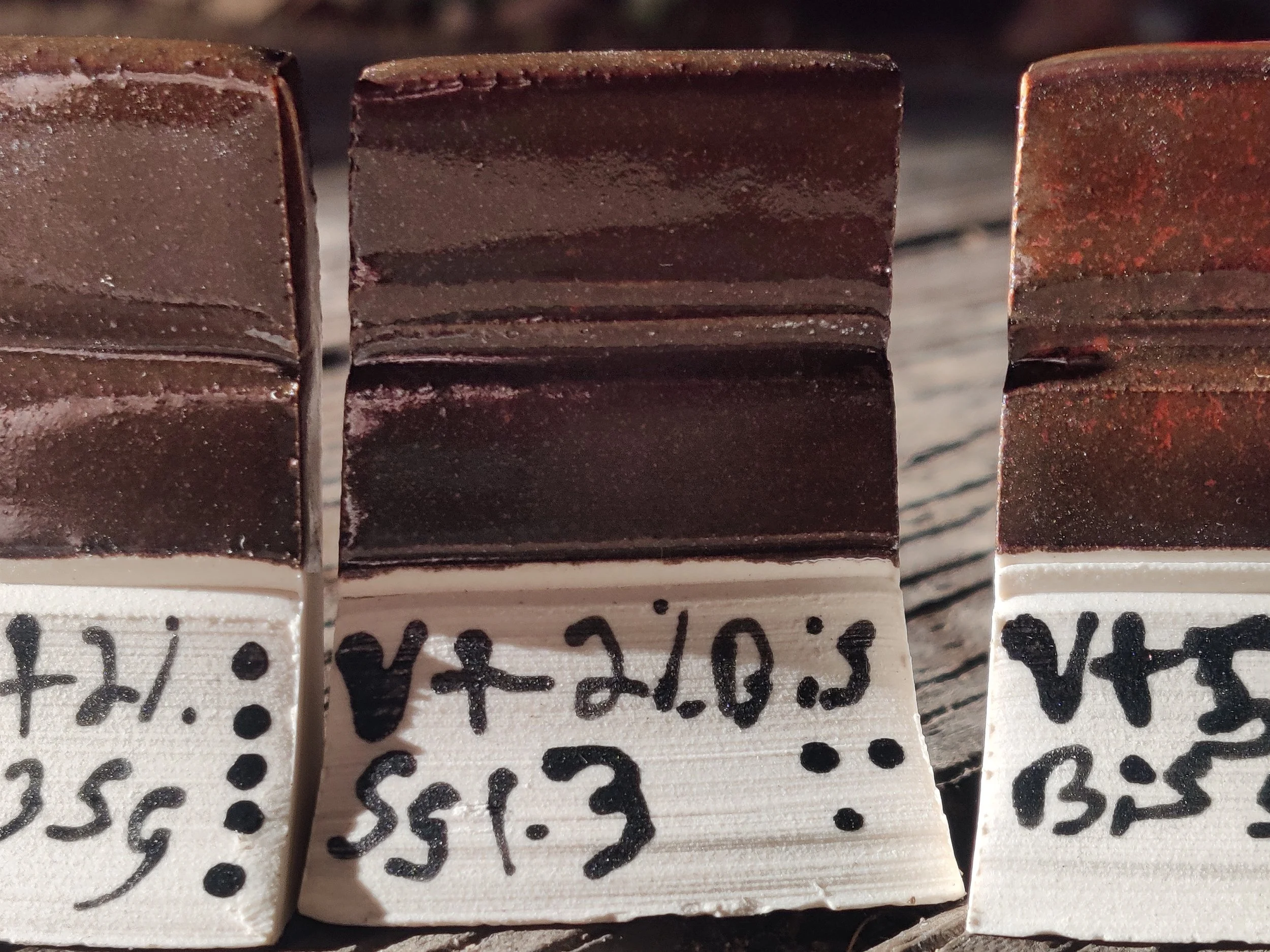Playing with bismuth in cone 6 glazes prt.1 (What are you!)
Sometimes…. I get bored. In this boredom I started playing with bismuth oxide. I needed to play with it because I do not know what it is or where it belongs in the UMF. I have read a bunch of articles on bismuth and none of them seem to have the info I want. Every article I read is either talking about it as a low temp flux or using it in larger amounts to create low fire “luster” type glazes; None of which I am interested in. I want to know what it will do at cone 6. Many notes claim is “stiffens up glazes”, what dose that mean…. Less running? One person even said it turns in to a toxic gas around cone 6……? Ok, well people are using it and surviving so that can’t be all true…. one of my most consistent books (The Potters dictionary by Frank and Kanet Hamer) says the organics burn off at low temp (see below picture) BUT THAT’S DAMN NEAR EVERYTHING IN THE KILN, ORGANICS DON’T SURVIVE THE KILN SO WHAT DOSE THIS MEAN!>!?!?
Page 25 from The potters dictionary by Frank and Janet Hamer.
I just need to test it at this point. I took one of my reddish glazes and added it in 1-8% jumping a few percentages as I go. I took Van gilders Kaki red from a John Britt book and added bismuth. I have experience with this glaze as it was one of the prime glazes I ran across when looking for a good cone 6 oxidation red. Here is the recipe to Van Gilders Kaki red I used and my results. This glaze works fine but clearly needs more metal flux to work. I do want to clarify, I am not using Bismuth trioxide or substrate; I am using Bismuth oxide.
Only this different is the Yellow iron oxide. Original recipe has Red iron oxide.
Van Gilders Kaki Red +2% bismuth oxide
Cone 5
SG: 1.3
Application: 5 second dip
Clay : bmix
Notes: little to no change. Keep in mind I have my own version of this glaze that usually has Gerstly borate. This one does not.
Van Gilders Kaki Red + 2% bismuth
Cone 5
SG: 1.3
Application: 3 second dip
Clay : bmix
Notes: had to test a 3 second dip just to make sure. Still nothing.
Van Gilders Kaki Red + 5% bismuth oxide
Cone 5
SG: 1.3
Application: 4 second dip
Clay : bmix
Notes: I see a little red now.
Van Gilders Kaki Red + 8% bismuth oxide
Cone 5
SG: 1.3
Application: 5 second dip
Clay : bmix
Ok. It’s clear this is getting us nowhere. Let’s try something els.
Van Gilders Kaki Red + 8% bismuth oxide
Cone 6
SG: 1.3
Application: 5 second dip
Clay : bmix
I retested it and held it at cone 6 for 10min. Now we see red but not the kind I like.
At this point I got tired of this line test game. When I enter it in to the UMF on glazy it puts it in to the metal fluxes. So, Let’s treat it like one. I made up a whole new glaze and put a relatively large amount of bismuth in. I took one of my red glazes (Red XIII) and put 11% bismuth in. Keep in mind this glaze turns red without the bismuth. Unfortunately I can’t give you the recipe but I can show you my results.
First let’s get a feel for the glaze without bismuth.
Red XIII
Cone 6 ox
Application : 4 second dip
SG: 1.32
Viscosity : not tested
mostly stable.
Notes: good glaze tbh, I just never use it.
Red XIII on dragon fruit
Cone 6 ox
Application : 4 second dip
SG: 1.32
Viscosity : not tested
Clay: dragon fruit mixed with blue porcelain.
mostly stable.
Red XIII
Cone 6 ox
Application : 2 second dip
SG: 1.32
Viscosity : not tested
clay: b-mix tile
mostly stable.
I’m showing you the same glaze over and over again so you can get a beat on how this glaze works with no Bismuth whatsoever. Now let’s look at the same glaze but with bismuth.
Red XIII + 11% bismuth
SG: 1.4
Clay: bmix
Application : 4 second dip
Cone : ^5 ox ( small kiln)
Red XIII +11% bismuth
SG: 1.4
Clay: bmix
Application : 3 second dip
Cone : ^5 ox ( small kiln)
Notes: Ignore the word lotus, I mixed up the tile. This is Red XIII
Red XIII + 11% bismuth
SG: 1.4
Clay: bmix
Application : 5 second dip
Cone : ^5 ox ( small kiln)
Even at 11% I see little change in movent. If you look close you can see a little crystal formation. This makes me suspicious of the UMF putting it in the Metal flux category. If it were we would probably see some running at +11% on top of a glaze that was already fully melted and pearling at cone 5. Imagine if you added 11% lithium to a glaze that was already maturing at cone 5; You think that would stay stable?! I don’t know. Maybe something like lithium is way more powerful then bismuth. I do not have enough experience to know.
Here is what I think. This is my first test with bismuth but if 11% is not melting an already fully melted glaze then it can’t be that strong. Do I have proof of this?, no. Nothing more than my tests and a small hypothesis. On top of that it’s expensive. You can just use other metal fluxes that are cheaper. On top of all of that it doesn’t seem to change anything color wise in any of my tests either.
I will fully admit, this is part 1, I need to experiment with this more in other glazes to say that full confidence BUT 11% DID ALMOST NOTHING?! I will retest this in the future with other glazes. Keep in mind I am using bismuth oxide.
In the end Almost all the info I can find from potters (even in person) is either refuted online, the info online doesn’t make sense, or whenever I ask for tests or proof people tell me “ oh, that’s just what I have read -_-” I personally do not think it’s a powerful flux, stiffener, or color enhancer and whatever magic this mineral provides is not providing it at 11% as an additive then I do not know what how it is to be used. One day I hope I see a real test at cone 6 or 10 that proves me wrong but for now I do now think it’s made for cone 6 or it’s not what the current literature says it is.